Lithium-ion battery auxiliary materials: type of binder, bonding mechanism, synthesis method, development trend
The raw material ratio of a complete lithium-ion battery must include active material, conductive agent, binder, solvent, and additives, and the binder plays an active material and foil, active material and active material. The effect of bonding between the active material and the conductive agent, though used in small amounts, is irreplaceable. In the following, we will make an exchange with you on the type of binder, the bonding mechanism, the synthesis method, and the development trend.
1. Oil binder PVDF:
1) Introduction: PVDF is the most commonly used oily binder in the lithium-ion battery industry. It is a kind of non-polar chain polymer binder. It is characterized by its strong anti-oxidation and reduction ability, good thermal stability, and easy dispersion, but it requires the use of N-methylpyrrolidone (NMP) as the solvent. The volatile temperature is relatively high, there is a certain degree of environmental pollution, and the price is expensive. Obvious deficiencies include relatively high Young's modulus, between 1-4GPa, the flexibility of the pole piece is not good enough; PVDF absorbs water and the molecular weight decreases, the viscosity becomes worse, so the humidity requirement for the environment is relatively high; for ions and electrons Insulation, there is a certain degree of swelling in the electrolyte, and with lithium metal, LixC6 exothermic reaction at a higher temperature, the battery's safety is unfavorable.
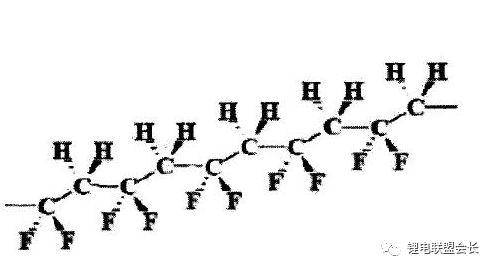
2) Bonding mechanism: conventional PVDF, the main mechanism of action is van der Waals force, which is the intermolecular force acting as a bond. Some modified PVDFs have two parts of their action mechanisms, one part is the van der Waals force brought by high molecular weight. On the other hand, it is due to the effect of the chemical bond between the foil and the modification.
3) Synthesis methods: The current synthesis methods include suspension polymerization and emulsion polymerization
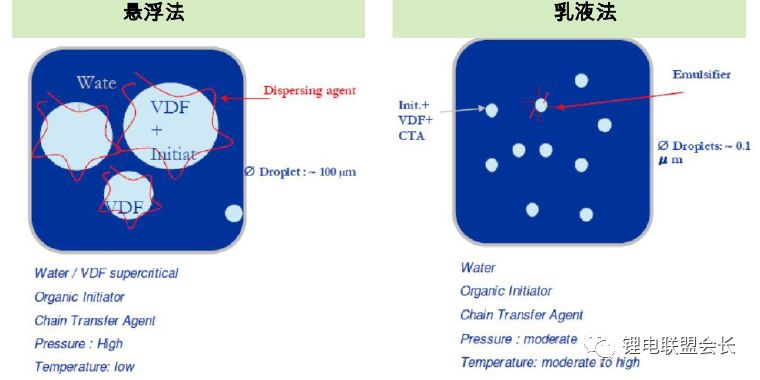
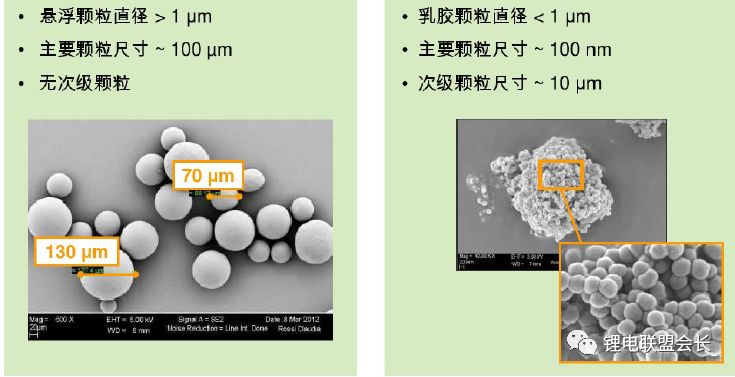
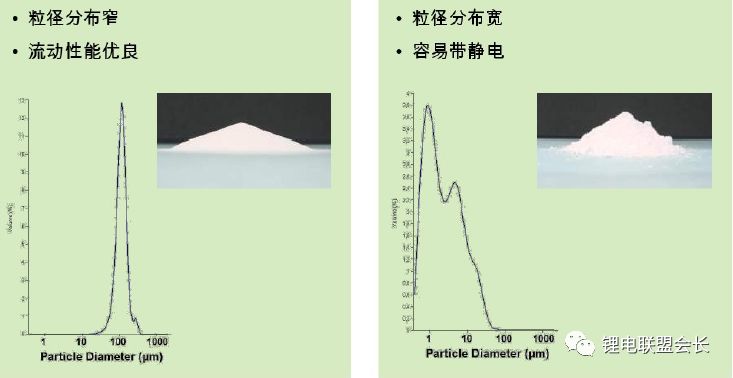
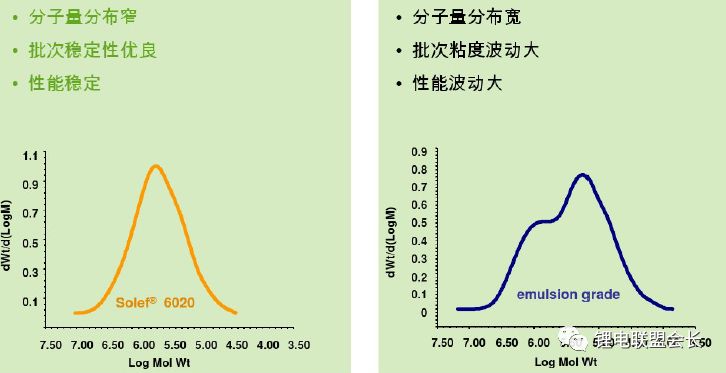

For different cathode materials, different methods can be used to synthesize PVDF, and it must be combined with the corresponding homogenization process to achieve a good result.
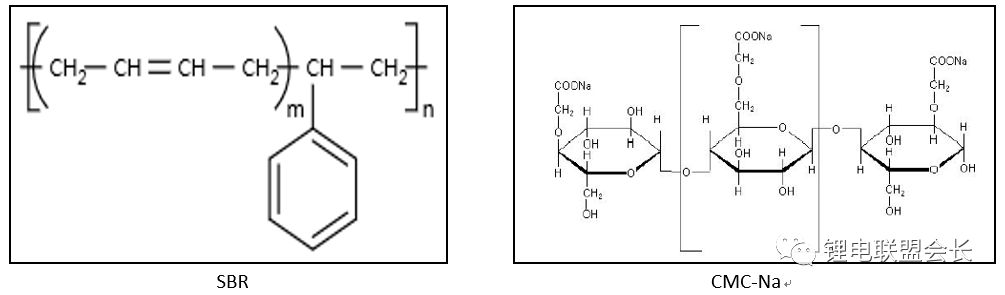
2. Water-based adhesive SBR, CMC-Na:
1) Introduction: SBR is the most widely used water-based adhesive, SBR is the English abbreviation of styrene-butadiene rubber, is easily soluble in water and polar solvents, has a high bond strength and good mechanical stability and Operable, used in the battery industry as a binder, the binder works well and the quality is stable
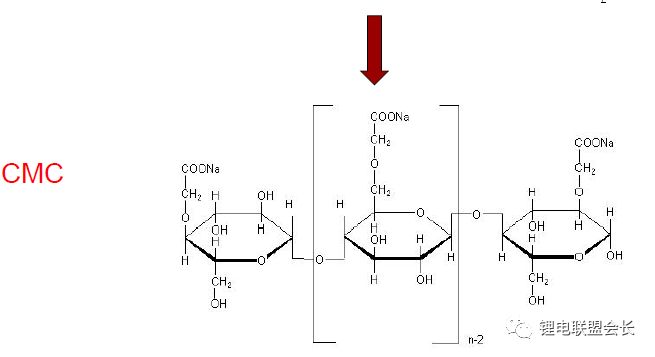
CMC-Na: Sodium carboxymethyl cellulose is the most widely used and most used type of cellulose in the world today. It is a cellulose derivative with a degree of glucose polymerization of 100 to 2000 and a relative molecular weight of 242.16. White fibrous or granular powder. Odorless, tasteless, odorless, hygroscopic, insoluble in organic solvents.
2) Adhesion mechanism: SBR surface groups undergo condensation reaction with the copper foil surface groups to form chemical bonds. The SBR emulsion itself is a product of a balance of hydrophilicity and hydrophobicity. On the one hand, the graphite is organically bound by hydrophobicity, and on the other hand, a condensation reaction occurs between the hydrophilic group and the surface group of the copper foil. The CMC-Na as a stabilizer, suspension dispersant, SBR has an auxiliary bonding effect, but also allows SBR more uniform dispersion, while the use of space charge repulsion to ensure the stability of the entire system.
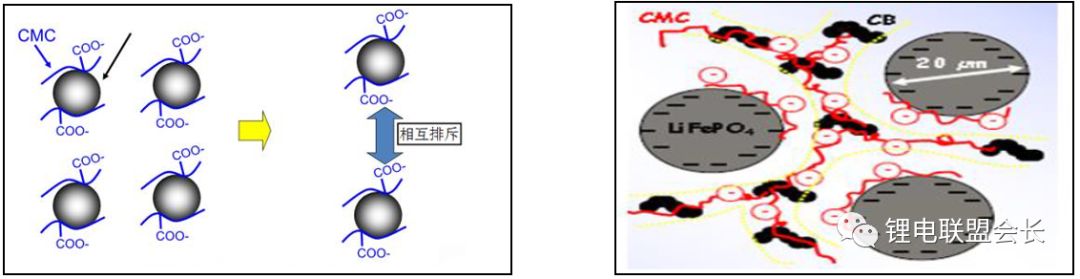
What needs to be explained here is that the joint use of SBR and CMC is a long-term cumulative result, and the roles are complementary and indispensable.
CMC and SBR are complementary to each other in the cathode negative electrode of a lithium battery, and they are indispensable, which is the result of long-term practice accumulation in the industry. If pure CMC is used as the adhesive, the condition is that the thickness of the pole piece is relatively thin and the rolling process is not carried out or the compaction density of the pole piece is not high. In the actual pole piece, the graphite pole is required due to the energy density requirement. The sheet must be rolled, and the compaction density is high. In this case, the CMC adhesive cannot be used alone, because the CMC is brittle, the structure collapses after rolling, and the pole pieces fall out of powder and cannot be used; SBR can not be used alone as a binder, because it is difficult to prepare a slurry, SBR does not have a suspension dispersion function, the slurry will precipitate it, while too much SBR will make the pole piece swell in the electrolyte; and CMC and Simultaneous use of SBR can basically solve the above-mentioned problems, because the graphite material itself is not hydrophilic, it is difficult to disperse in the water system. One of the functions of using the CMC is as a dispersant, dispersing graphite and conductive additives, and the CMC is in water. The gel will form, making the slurry thickened. When applied in large scale, because of the presence of the gel structure, it can both retain water and stabilize the slurry, and can maintain the uniformity of the slurry within a certain period of time. It is conducive to large-scale production; at the same time, SBR is introduced, because SBR emulsion is soluble in water, SBR itself is a flexible material, has a good bonding performance, so that the pole piece will not fall powder under the high pressure conditions. Rolled pole pieces also have high bond strength.
3) Synthetic methods and detection parameters
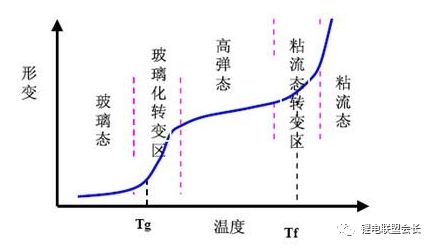
Synthetic method of SBR: SBR is synthesized from 1,3-butadiene (CH2=CH-CH=CH2) and styrene (C6H5-CH=CH2). There are generally two methods of emulsion polymerization and solution polymerization; Adjust the ratio of the two to obtain a series of different cross-linking degrees and different glass transition temperatures (one is the temperature at which the deformation takes place, and the other is the transition temperature from the glassy state to the highly elastic transition temperature).
Emulsion polymerization: In the early stage, potassium persulphate was used as an initiator to prepare butadiene and styrene by free radical emulsion polymerization at 50°C, commonly known as hot glue, and there is still a small amount of production. Since the 1950s, the polymerization temperature has generally been reduced to 5°C in industrial production. The basic product is SBR1500, commonly known as cold glue. Cold glue is produced by dispersing butadiene monomers in aqueous emulsions of rosin soaps or fatty acid soaps as emulsifiers, using thiols as molecular weight regulators, and adding oxidation consisting of organic peroxides, ferrous salts, and activators. - Reduction initiates the system for free radical polymerization. Emulsion polymerization is carried out continuously in a plurality of tanks in series, and the conversion rate is controlled to about 65%. Unreacted butadiene and styrene were successively removed using a horizontal flash tank and a distillation column, and refined and reused. The unreacted monomer copolymer emulsion is coagulated with sodium chloride, calcium chloride and acid, and the resulting rubber is separated from the whey by a vibrating sieve, and then dehydrated and dried to obtain a finished product. Compared with hot glue, cold glue has low degree of branching and cross-linking, gel and low-molecular-weight content is greatly reduced, performance is significantly improved, so the hot glue is basically replaced.
Solution polymerization: Styrene butadiene rubber synthesized in a non-polar solvent with butyllithium as a catalyst. The solution polymerization styrene-butadiene rubber is divided into two groups, block copolymers (thermoplastic rubbers) and random copolymers. Solution polymerized styrene-butadiene rubber tends to spontaneously form polystyrene blocks during the copolymerization process. In order to synthesize a copolymer in which the styrene is randomly distributed on the main chain (that is, does not contain a polystyrene block), continuous polymerization may be performed. Additional monomers, high temperature polymerization at 90-150°C, and the addition of ethers, tertiary amines, phosphites, sulfides, or surfactants as random agents. The molecular weight distribution of solution-polymerized atactic styrene-butadiene rubber is narrower than that of emulsion-polymerized styrene-butadiene rubber, and the degree of branching is also low. In order to reduce the cold flow tendency of the raw rubber, it is necessary to add divinylbenzene or tin tetrachloride as a cross-linking agent during the copolymerization process so that a small amount of cross-linking occurs between polymer molecules. It is also possible to blend copolymers having different molecular weights to broaden the molecular weight distribution. The content of the top-1,4 isomers of the solution-polymerized atactic styrene-butadiene rubber is 35%-40%, which is better than the emulsion-polymerized styrene-butadiene rubber, such as wear-resistance, flexure, rebound, heat generation, etc., and shrinkage after extrusion. Small, can replace the emulsion styrene butadiene rubber in general, is particularly suitable for the production of light-colored or transparent products, can also be made of oil-filled rubber.
CMC synthesis method:

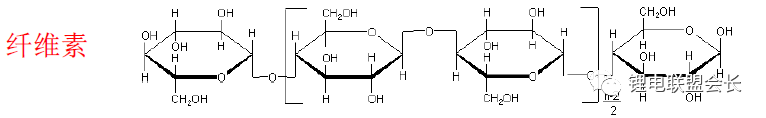
Raw materials mainly come from natural cotton, straw, wood, etc. The key parameters are viscosity, degree of substitution, purity and other parameters.
The degree of substitution (DS) refers to the average hydroxyl value on the anhydroglucose unit. If all three hydroxyl groups are substituted, then the DS theoretical maximum is 3.0. The figures above are the states when the degree of substitution is 0.7 and 1.2. The higher the degree of substitution, the stronger the hydrophilicity, and the easier it is to absorb water. The CMC acts as an adhesion aid and dispersant for the negative electrode.
3. Future trends and directions
With the continuous development of lithium-ion technology, more and more new types of adhesives have begun to enter the field of vision. Polyacrylic acid (PAA), polytetrafluoroethylene (PTFE), polyimide (PI), etc. In-depth research was conducted and some results were achieved. With the continuous popularization of silicon-carbon materials, high-temperature resistance, good tensile strength, composite conductive agent with both conductivity and adhesion have begun in-depth research. In the future, the adhesive needs to be custom-designed, and according to the comprehensive factors such as the surface morphology, state, and functional group of the material, the existing adhesives can be custom-developed for the appearance and surface conditions so as to continuously meet the high energy density. Battery requirements.
references:
1. Lithium-ion battery PVDF binder research data
2. Optimization of environmentally friendly SBR synthesis process
3. High specific energy negative electrode binder for lithium ion batteries
4. Lithium-ion battery binder industry introduction
5. Lithium-ion battery binder research progress
6. China's lithium battery binder market development status and market prospects
7. Ashland CMC for LI-B
Safety Light Curtain,Safety Curtain,Laser Safety Light Curtain,Safety Optic Light Curtain,Security Light Curtain,Press Brake Safety Light Curtains
Jining KeLi Photoelectronic Industrial Co.,Ltd , https://www.sdkelien.com
